press release
Sonoscanner Achieves EU MDR Certification, Demonstrating Its Commitment to Quality and Safety of Its Ultrasound Devices!

Sonoscanner, a French company specializing in the manufacturing of ultrasound devices, is proud to announce its compliance with the European Medical Device Regulation (MDR). As a leader in clinical ultrasound in France, Sonoscanner is among the first companies to receive this prestigious recognition.
Sonoscanner, a leader in the design and manufacturing of advanced ultrasound devices, is proud to announce that it has just obtained the European Medical Device Regulations (MDR) certification. Achieving this compliance is a recognition of the importance that the company places on the quality of its ultrasound devices and the rigorous adherence to production processes.
Obtaining the MDR certification is also a mandatory milestone for the company’s international development. For Sonoscanner, a major player in the development and promotion of French MedTech globally, this opens new growth opportunities.
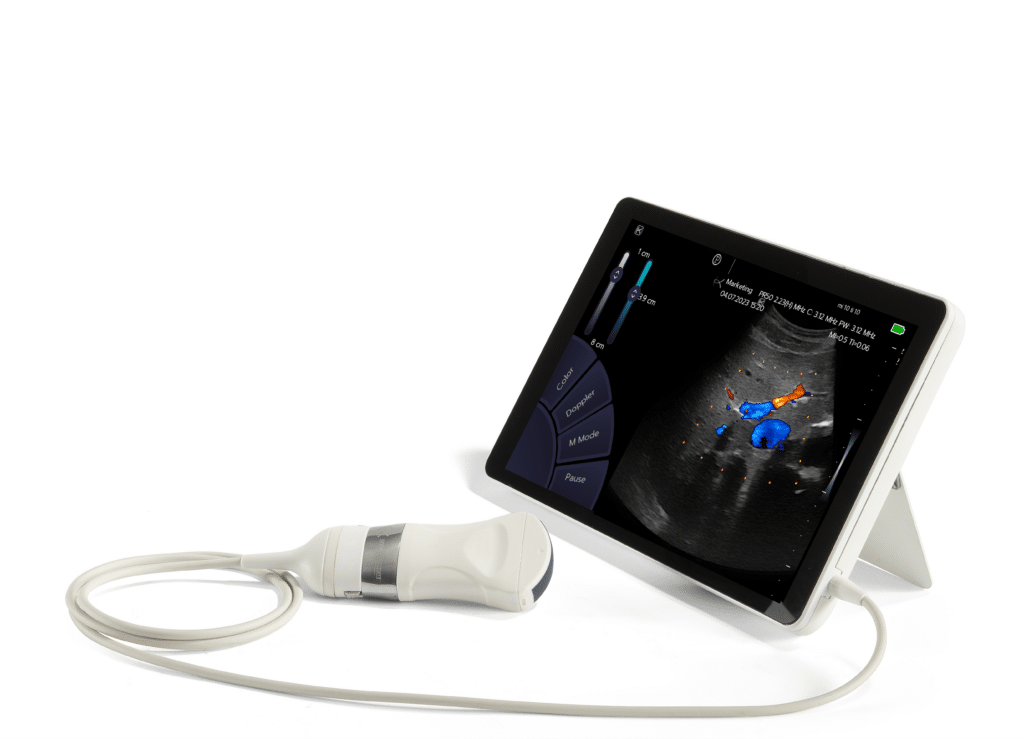
For over 20 years, Sonoscanner has built its reputation in the ultrasound market by offering products that combine innovation, reliability, and simplicity, thanks notably to a constant commitment to research and development. The latest product from the French company, the ultraportable U-Lite PRO ultrasound device, is proof of this: featuring exceptional image quality, this revolutionary device is the first to integrate all Doppler functionalities in a portable format, opening new perspectives in the field of medical imaging.
Étienne Richard, CEO of Sonoscanner, expressed his satisfaction with this new milestone for the company: “Achieving MDR compliance is a major step for Sonoscanner. It demonstrates our ongoing commitment to the excellence and safety of our products, as well as our determination to meet the growing market demands for high-quality medical devices.”
Through its swift compliance with the MDR regulation, Sonoscanner reaffirms its commitment to its clients, partners, and healthcare professionals by providing innovative, reliable, and safe ultrasound devices that meet the strictest regulatory and quality requirements. This certification comes at an opportune time as the company prepares to unveil a new ultrasound device at the beginning of the summer. Achieving this certification also heralds an acceleration of Sonoscanner’s international growth.